Electronic properties of gas phase systems
Electronic properties of gas phase systems
Contact persons: Michele Alagia, Monica de Simone, Marcello Coreno, Cesare Grazioli
|
The electronic structure of any matter determines its most physical and chemical properties. It can be studied with spectroscopic methods: one subjects matter, for instance, to electromagnetic radiation and observes how it responses. We perform our studies by directing monochromatized synchrotron radiation on isolated molecules or atoms and by detecting angular and energy distributions of any particles that are ejected: electrons, singly or multiply charged ions and photons, sometimes in coincidence with each other. These processes can be understood best in gaseous samples. We carry out research within the Research Group of the Gas Phase Photoemission beamline (Elettra- Sincrotrone Trieste, CNR-IOM and CNR-ISM). The beamline delivers highly monochromatic radiation in the photon energy range 14-900 eV. Lowest photon energies are suited to studies of valence electron transitions, while the high energy part allows us to induce 1s electron transitions in atoms such as C, N and O. |
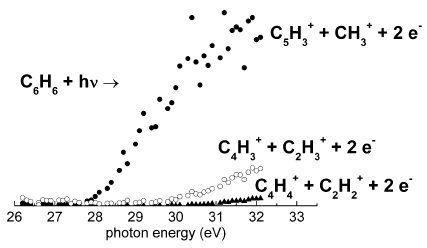
Alagia et al [Phys. Chem. Chem. Phys. 13, 8245 (2011)] used a time-of-flight mass spectrometer to study dissociative double ionization of benzene by VUV synchrotron radiation. The threshold energies of the main dissociative processes were characterized by exploiting the photoelectron-photoion-photoion coincidence technique. |
As an example of valence ionization studies, the electronic structure of iron phthalocyanine (FePc) was examined within a joint theoretical–experimental collaboration [J. Chem Phys. 134, 074312 (2011)]. Particular emphasis was placed on the determination of the energy position of the Fe 3d levels in proximity of the highest occupied molecular orbital. Photoelectron spectroscopy measurements were performed on FePc in gas phase at several photon energies between 21 and 150 eV. In the core region, S 2p photoionization in the SF 6 molecule has attracted much attention because its cross section displays very intense shape resonances. In a combined experimental and theoretical study, Stener et al [J. Chem. Phys. 134, 174311 (2011)] proved that the effect of the shape resonances extends even up to 80 eV above the S 2p ionization thresholds. In another study of the SF 6 molecule, Auger decays of the S 2p -1 states and of its first shake-up state, which is characterized by an electron configuration S 2p -1 val -1 virt 1 , could be separated using electron-electron coincidence spectroscopy [J. Chem. Phys. 134, 094308 (2011)]. We have studied O 1s excitation and ionization processes in the CO 2 molecule using UV-visible fluorescence spectroscopy and VUV photon-photoion coincidence technique [J. Phys. B 44, 165103 (2011)]. Detected UV and VUV photons are created when the final states of Auger decay dissociate into valence-excited neutral or ionic fragments. Remarkably, the fluorescence yield of a transition in the neutral O atom displays all the single and double excitation features seen in the O 1s absoption spectrum of CO 2 . In another study, Kivimäki, Alagia and Richter [Chem. Phys. Lett. 531, 252 (2012)] exploited x-ray emission-photoion coincidence spectroscopy to study how the CO 2 molecule dissociates when diverse O 1s hole states decay via soft x-ray emission.