Electronic and paramagnetic properties of open shell molecular thin films
Electronic and paramagnetic properties of open shell molecular thin films
Contact person: Maddalena Pedio
|
|
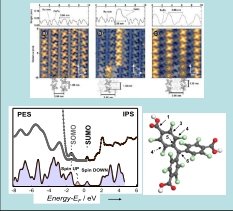
Up: STM Nanochains of FePc on Au(110) as a function of coverage a) 0.5, b 0.7, c) 0.8 ML. Bottom Right: Molecular structure of radical PTMTC. C grey; Cl green; oxygen red; H white. Bottom Left: PES-IPS spectra of thin films of PTMTC the SOMO and SUMO respectively. Bottom: calculated Density of States (DOS) of the spin up (red shadow) and spin down (blue shadow) of the isolated molecule.
|
For Transition Metal complexes the interconnection between the structural phases of ordered FePc-nanochains self-assembled on Au(110) and the paramagnetic properties has been enlighten. [1] The chain assembly is driven by the molecule−molecule interaction and the chains interact with the Au nanorails via the central metal atom, while the chain−chain distance in the different structural phases is primarily driven by the redistribution of the Au substrate. In case of FePc and CoPc this hybridization leads to a decreasing of the paramagnetic properties. The magnetic moment is recovered only for thin films aggregations. Purely organic free radicals appear suitable candidates towards applications thanks to the small spin orbit-coupling and hyperfine interactions that allow for long spin relaxation times. In case of three dimensional radical PTMTC and the presence of a Single Occupied Molecular Orbital (SOMO) and its unoccupied counterpart (SUMO) are clearly detectable as frontier orbitals in combined Photoemission (PES) and Inverse Photoemission (IPS) data [2]. Recently we found that in case of PTMTC deposition on Au(111) the paramagnetism of the organic radical is preserved while it appears partially quenched on Ag(111). This antithetic behaviour is explained on the basis of a detailed study of the interfacial electronic properties: The spin loss and preservation for such organic radicals could not have been demonstrated without looking inside the interface.
Collaborators: M. Kumar, Cinzia Cepek, S. Fabris, S. Fortuna, J. Fuji, G. Rossi, A. Calzolari (Nano-CNR), J. Veciana (ICMAB-ICSI Barcelona), V. Mugnaini (KIT Karlsruhe, Germany) Coll. PRIN SurfTalo coord. M.G. Betti
[1] M G Betti, P.Gargiani, Carlo Mariani, R. Biagi, J.Fujii, G.Rossi, A. Resta, S. Fabris, S. Fortuna, X. Torrelles, M. Kumar, M. Pedio, Langmuir 2012 DOI: 10.1021/la302192n; P.Gargiani, G. Rossi, R. Biagi, V. Corradini, M. Pedio, S. Fortuna, A. Calzolari, S. Fabris, J. Criginski Cezar, N. Brookes, M. G. Betti Phys. Rev B, 87 165407 (2013).
[2] F. Grillo, V. Mugnaini, M. Oliveros, S. M. Francis, D-J. Choi, M. V. Rastei, L. Limot, C. Cepek, M. Pedio, S.T. Bromley, N.V. Richardson, J-P. Bucher, J. Veciana, J. Phys. Chem. Lett., 3 1559-1564 (2012).