Looking Inside the Perchlorinated Trityl Radical/Metal Spinterface through Spectroscopy
Persistent and stable organic molecules with an open-shell electronic configuration have long been known and extensively studied mainly in solution. Only recent is instead the study of organic radicals as laterally self-assembled monolayers immobilized on substrates towards their application in devices, e.g. in organic spintronics [1]. To exploit this field of applied research, the knowledge of the electronic properties of the organic side of the organic radical/metal interface is of primary importance. We report on a spectroscopic multitechnique approach (photoemission PES, inverse photoemission IPS and near-edge X-ray absorption fine structure NEXAFS spectroscopies) to study the thin film properties and the metal/radical spinterface formed by a perchlorinated trityl radical derivative (PTMTC) and either gold or silver. The spectroscopic fingerprint of the PTMTC paramagnetic properties could be determined by comparison with the diamagnetic precursor αH-PTMTC and by DFT calculations (Figure 1, 2). Thanks to the our approach, we could gain unprecedented insight into the radical−metal interacAon with metal substrates, namely Au(111) and Ag(111), and how this latter perturbs the spin polarization and consequently the magnetoelectronic properties of the radical adlayer. Knowledge of the factors influencing the spinterface is an essential tool toward the tailoring of the properties of spin-based electronic devices. The presence of the Single Occupied Molecular Orbital (SOMO) and the SUMO (NEXAFS). Through the comparison with Density Functional Theory (DFT) calculations we provide a consistent framework for the microscopic understanding of the preservation of the paramagnetism of PTMTC on metal surfaces and its interaction with them, which can be antithetical depending on the metal support.
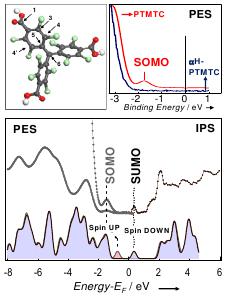
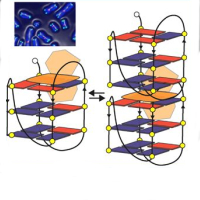
Figure 1. Top part. Left: optimized geometry of the radical derivative tri-para-carboxylic
perchlorotriphenylmethyl (PTMTC) in the gas phase. Right: PES of PTMTC (red) and its non
radical precursor (blue) αH-PTMTC. Bottom part. Top: Combined PES and IPS spectra of
PTMTC thin film on Au(111). The peaks related to the Single Occupied Molecular Orbital
(SOMO) and its unoccupied counterpart (SUMO) are labeled. Bottom: calculated Density of
States (DOS) of the spin up (red shadow) and spin down (blue shadow) isolated molecule.
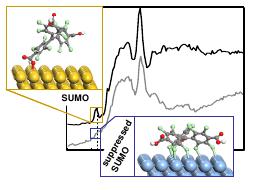
Figure 2 Proposed models of the interfacial PTMTC on Au(111) (yellow atoms) and
Ag(111) (blue atoms) and the C K edge NEXAFS spectra of 1ML on the two substrates.
DFT calculations combined with a multi-technique approach, prove that the open-shell
electronic (SOMO-SUMO) configuration of a perchlorinated trityl radical is preserved on
Au(111), while it is partially quenched on Ag(111) surface due to chlorine mediated
strong interactions with the surface.
Veronica Mugnaini, Arrigo Calzolari, Ruslan Ovsyannikov, Antje Vollmer, Mathieu Gonidec,Isaac Alcon, Jaume Veciana, and Maddalena Pedio, J. Phys. Chem. Lett., 6 (2015) 2101−2106. DOI