Glass transition – structural arrest: molecular liquids, polymers, glues
Glass transition – structural arrest: molecular liquids, polymers, glues
Contact persons: Lucia Comez, Silvia Caponi
| The research concerns the multiple aspects of the glass transition phenomenon. The formation of a glass can be produced both by physical and chemical paths. The first is the usual way to generate a glass: a liquid in a metastable state is cooled or compressed so as to avoid crystallization; the second represents an alternative practice, i.e. the chemical vitrification: a process involving progressive polymerization of the constituent molecules via the formation of irreversible chemical bonds. The formation of most of the materials used in engineering plastics and the hardening of natural and synthetic resins are based on chemical vitrification. Despite the differences in the molecular processes involved in chemical and physical vitrification, the detection of common ingredients is extremely challenging for the scientific community. |
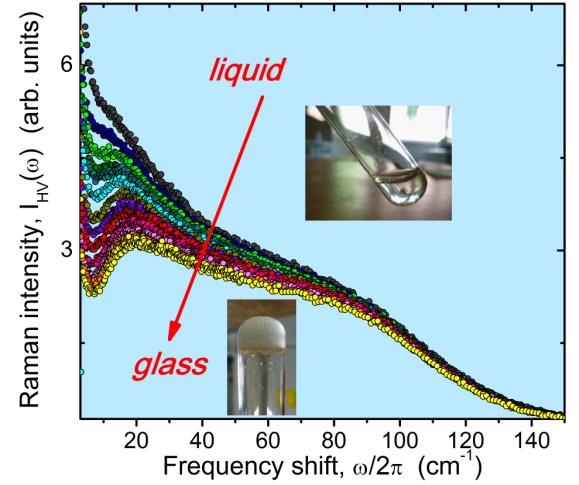
Fig. 1 Representative Raman spectra during a chemical vitrification. |
However, enormous efforts are necessary to explore the whole progression of the phase transformation. From an experimental point of view, the study of the glass transition requires experiments over a wide frequency band since the dynamical range of processes involved, when the system passes from the liquid to the amorphous state, is very huge (timescales from picoseconds to hundred of seconds). In addition, for achieving results that may have a universal character, several systems belonging to different categories, from conventional glass- formers to polymers capable of undergoing a chemical vitrification, should be considered. On this ground, thanks to the combined use of multiple techniques and the comparison among different systems (glycerol, vitreous silica and germania, epoxy resins), we have observed unexpected similarities in the slowing down of the dynamics and in the thermodynamical properties of the resulting glasses. Our research has been focused to explain such similarities that would improve general understanding of the glass transition and may unveil its universal nature. In particular, we have found that the obtained chemical and physical glasses share numerous features concerning the cooperative, the kinetic and the vibrational dynamics. In detail: (i) The slow dynamics (relaxation time >µs) has been investigated by photon correlation spectroscopy and explained in terms of bond–controlled configurational entropy reduction, identifying a common paradigm to physical and chemical variables; (ii) The fast dynamics (relaxation time ~ps), analyzed by means of Brillouin spectroscopy in different frequency domains, has shown intriguing features addressed by the mode–coupling theory for simple liquids, revealing that chemical bonding surprisingly, yet efficiently, realizes the mechanism of dynamical arrest described by the theory; (iii) The modifications of the vibrational density of states throughout the polymerization, studied by Brillouin and Raman spectroscopies, has been fully explained by the corresponding changes of the Debye level, in a similar manner as in densified glasses. In this last context, we have contributed to also demonstrate the validity of a strong connection, of a general nature, between some acoustic properties, as attenuation and longitudinal compliance, and the density of states of a system when it vitrifies.