Trapping of charged gold adatoms by dimethyl sulfoxide on a gold surface
The (111) face of gold, for its inertness and stability, is the most popular substrate adopted to support the growth of 2D supramolecular architectures. Despite being inert (“noble”) in its bulk form, gold exhibits rich catalytic properties and ligand chemistry when in the form of small clusters composed by tens of atoms down to single atom. In this work, we show that a simple polar molecule, dimethyl sulfoxide (DMSO, (CH3)2S), can trap the natively single gold adatoms available on the Au(111) surface, thus providing a new insight into the interaction of DMSO (and solvents in general) with gold and suggesting a novel motif for anchoring organic adlayers of polar molecules on metal substrates.
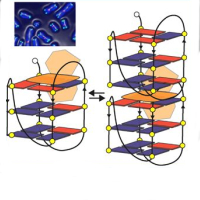
Low Temperature Scanning Tunneling Microscopy (LT-STM) images show that DMSO form complexes made of three or four molecules as shown in Figure a-d, namely symmetrical rectangle, asymmetric rectangle, chiral rectangle, and triangle, respectively. The configuration of such complexes (suggested by STM images and X-ray spectroscopic techniques), with DMSO molecules in the “inverted umbrella” geometry and with the O atoms located close to each other, should be extremely unfavorable due to the negative charge on the O atoms. Indeed, the Density Functional Theory (DFT) simulations indicate that these complexes are stable if, and only if, one or two charged gold adatoms are present at the center of the complexes linking the O atoms via a bond of ionic nature. These adatoms, even if present, are not visible in both STM experimental and simulated images.

Figure: STM image (center, 13 × 13 nm2) showing the various complexes that form on Au(111) after annealing to 273K. (a-d) Insets show high resolution details of complexes together with ball models of geometries obtained by DFT calculations, where the gold adatoms are painted in green to ease their identification.
References to original paper:
“Trapping of Charged Gold Adatoms by Dimethyl Sulfoxide on a Gold Surface”. Z. Feng, S. Velari, A. Cossaro, C. Castellarin-Cudia, A. Verdini, E. Vesselli, C. Dri, M. Peressi, A. De Vita, G. Comelli, ACS Nano 2015, 9, 8697–8709.
https://pubs.acs.org/doi/abs/10.1021/acsnano.5b02284